We offer consistent and reliable audit services by our expertise auditors globally
Our Services ensure that your operations and products meet the highest Quality, Compliance, and Regulatory requirements.

GxP Audit Services
We specialize in conducting GxP audits like GMP, GLP, GDP, Mock inspections, Gap analysis, and supplier audits to ensure your manufacturing processes and QMS procedures adhere to the industry’s best practices, guaranteeing product quality, safety, efficacy, and regulatory requirements.
Audit Services include
Supplier Qualification
API, Intermediates, Packaging Materials, and drug-device combination products.
CMO / CDMO
Conducts audits of Contract Manufacturing organizations of different dosage forms (Tablets, Capsules, Injectables, Ophthalmic, Nasal, Inhalers and drug device combination products)
GLP (Good Laboratory Practices)
Our GLP audits assist laboratories in adhering to stringent quality control measures, accurate data generation, and overall integrity in various scientific research and testing environments.
GAMP (Good Automated Manufacturing Practices)
We specialize in evaluating automated systems used in manufacturing processes, ensuring they meet regulatory requirements and maintain data integrity throughout the production cycle.
GDP (Good Distribution Practices):
With our expertise in GDP audits, we help organizations maintain the integrity of their supply chains, ensuring proper storage, transportation, and distribution of products while safeguarding their quality.
GCP (Good Clinical Practices)
Our GLP audits assist laboratories in adhering to stringent quality control measures, accurate data generation, and overall integrity in various scientific research and testing environments.
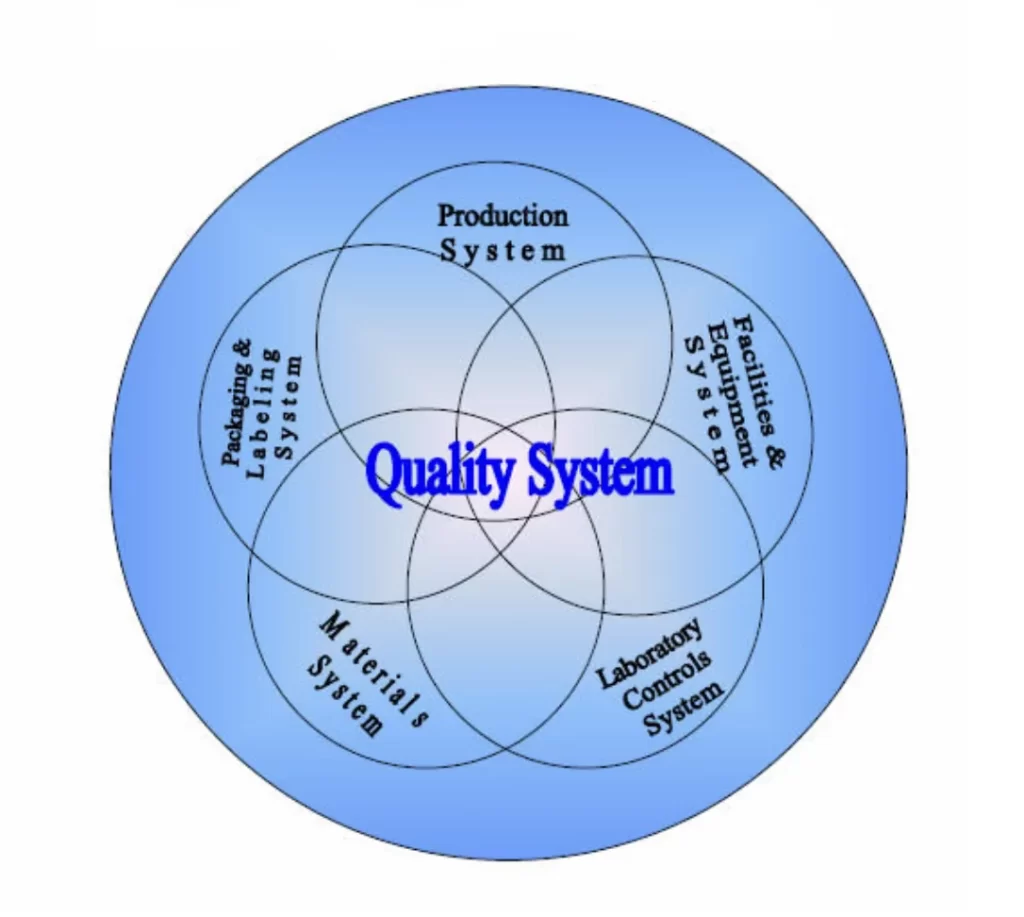
Quality Management System (QMS)
Our expert team will review the manufacturing investigations, Out of Specifications (OOS), Market complaints, CAPA and Risk assessments Comprehensively ensure adequacy.
Remediation
We provide a comprehensive response along with objective evidence demonstrating the corrective actions taken to non-compliant issues, 483 observations, regulatory actions, and warning letters.
Review the day-to-day operations and QMS notifications as committed to the agency.


Standard Operating Procedures (SOP)
We prepare the Standard Operating Procedures for your R&D, Technology transfer and commercial manufacturing (QMS, Production, QC and QA) Operations as per the regulatory requirements.
Training
We impart the training to the Operators, executives and quality leads on GMP, GLP, GDP, Investigations and Data integrity by demonstrating through with live examples.

At our core, we prioritize delivering consistent and reliable audit services that help our clients achieve compliance excellence. By choosing our expertise, you gain access to a global network of professionals dedicated to ensuring your operations align with the highest standards.

Contact Us
Contact us today to discover how our audit services can bring value to your organization on a global scale.
Address: AVR Homes, Flat no 402, Block 2, Sairam nagar colony, Miyapur, Hyderabad, Telangana, India 500049
Phone: +91 72076 86897
Email: Info@globalpharmaceuticalservices.com
